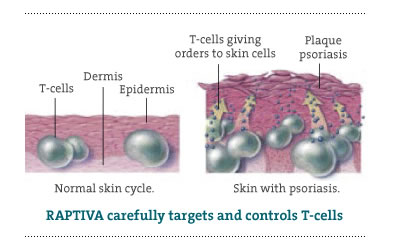
 | Generic Name: efalizumab Raptiva is a medicine given by injection and is used to treat adult patients (18 years or older) with moderate to severe plaque psoriasis. It is a therapeutic
antibody designed to selectively and reversibly block the activation and reactivation of T-cells that cause psoriasis. Between
5.8 and 7.5 million Americans are diagnosed with psoriasis, but of these only 1.5 to
1.9 million are considered to have a moderate to severe case of it. |
Psoriasis is a chronic skin disorder characterized by inflammated, red skin covered with silvery scales. Patches of circular to oval shaped red plaques that itch or burn raise up on the surface of the skin. It may appear on any area of the skin, but it is commonly found on the arms. Psoriasis is not contagious and can be inherited if it is present in family history. The U.S. Food and Drug Administration (FDA) approved
Raptiva in October 2003. It can be administered at home by the patients themselves via injection, once a week. It is prescribed based on body weight, so dosage may vary slightly. Clinical studies have demonstrated
that Raptiva reduced symptoms of psoriasis in just as little as four weeks. Side Effects Common side effects include:
|  Serious side effects include:
|
 | Generic Name: rituximab Background Rituxan is the first therapeutic anitbody approved by FDA for cancer treatment in November 1997. It is used to treat a type of cancer called non-Hodgkin's lymphoma. It can be used alone, with other cancer medicines, or with chemotherapy to treat other autoimmune, such as Rheumatoid Arthritis. This drug interferes with the growth of cancer cells by slowing their growth and slowing the rate of dispersion in the body. |
Non-Hodgkin's Lymphoma is a type of cancer affects the lymphocytes. These lymphocytes are white blood cells that are a part of the immune system. There are around 30 different types due to the different appearances of lymphoma cells viewed under the microscope. These variations of non-Hodgkin's lymphoma are characterized by enlarged lymph nodes, fever, and weight loss and can occur at any age.
Rituximab's first hint of discovery came from the Nobel prize
winning technique of discovering specific monoclonal antibodies (mAbs)
in mice cells. In 1979 Lee M. Nadler developed an mAb that attached to
the receptor site (CD20) of cancerous and normal B-cells. Once
attached, the mAb induces two or three of the immune system's four defense mechanisms, killing the B-cell and itself.
Because the stem cell in the bone marrows do not contain the CD20
antigen, new healthy B-cells can redevelop after treatment. Side Effects Common side effects include:
For real life changing stories from people who've taken Rituximab: Changed Lives | 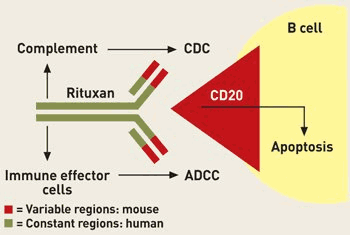 Rare/Unlikely side effects include:
|
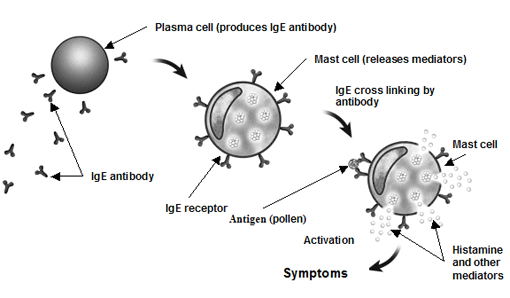
 | Generic Name: omalizumab Background Xolair is a proscription drug that was founded by Genentech and approved by the FDA on February 21, 2007. It is used
for children and adults over the age of twelve. Xolair is used to treat moderate to severe
asthma by selectively binding
to immunoglobulin E (IgE) of air-born allergens to help stop asthma
attacks and symptoms before they start. |
It is administered through injection by a trained
professional, but also by the patient themselves. It usually given to patients if other asthma medications have not made progressive treatment. Small dosages are recommended, as large dosages can be harmful. This drug will dramatically reduce
the number and the severity of asthma attacks, but
small asthma attacks may still occur. Xolair has
not been proven to treat any other allergy conditions besides allergic asthma.
Asthma
is a disease that affects the body's respiratory system (airway and lungs). It is inherited or triggered by environmental factors such as over-exertion during exercise or during a cold. Allergic asthma is caused by allergens in the air and can be triggered by dust particles, mold, pollen, pet dander,
and scented fragrances.
Side Effects
|
|
A severe reaction to Xolair called anaphylaxis has occurred in rare cases. Signs and symptoms include:
- Wheezing,
shortness of breath, cough, chest tightness, or trouble breathing
- Low
blood pressure, dizziness, fainting, rapid or weak heartbeat, anxiety, or
feeling of "impending doom"
- Flushing,
itching, hives, or feeling warm
- Swelling
of the throat or tongue, throat tightness, hoarse voice, or trouble
swallowing